Our research activity is based upon organic synthesis that can afford new organic materials utilized in optoelectronic devices, such as organic field-effect transistors (OFETs), organic solar cells (organic photovoltaics, OPVs), and organic thermoelectric devices (OTE). To this end, our team develops new organic materials, which can be designed and synthesized in order to have appropriate molecular and electronic structures for target functionalities. Our recent achievements are: 1) high-performance molecular semiconductors applicable to OFETs with the high mobilities, 2) new non-fullerene acceptors and their OPVs showing high power conversion efficiencies, and 3) new molecular design strategies to control packing structures of organic semiconductors.
Development of high-mobility organic semiconductors by controlling molecular arrangement
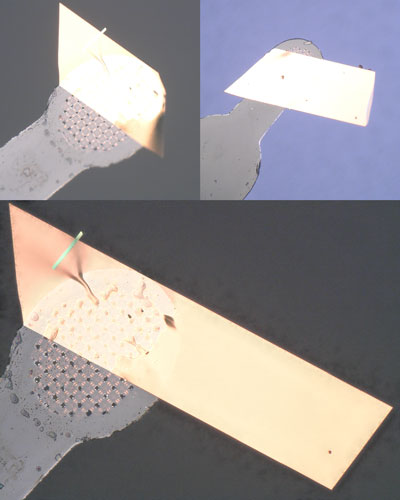
Solid-state properties of organic semiconductors, e.g., carrier mobility, are largely dependent not only on the molecular structure but also packing structure and molecular orientation in the solid state. However, it is very difficult to predict and control the crystal structure at the stage of molecular design, and the development of methodologies for controlling the crystal structure of organic semiconductors is an important issue. We have found that it is possible to lead to a crystal structure suitable for high mobility by introducing a simple substituent such as a methylthio group at an appropriate position in the organic semiconductor skeleton. For example, When four methylthio groups were regio-selectively introduced into pyrene that crystalizes into a sandwich herringbone structure, the crystal structure changes dramatically into a brickwork structure, which enables two-dimensional conduction. The carrier mobility evaluated by using single-crystal field-effect transistors was higher than 30 cm2 V-1 s-1, which was among the highest for organic semiconductors.

Development of high-mobility organic semiconductors by controlling molecular arrangement
Control of packing structures of thienoacene-based organic semiconductors
Solid-state properties of organic semiconductors, e.g., carrier mobility, are largely dependent not only on the molecular structure but also packing structure and molecular orientation in the solid state. The packing structures of representative organic semiconductors, e.g. acenes and thienoacenes, are classified into a herringbone packing, which is characterized with a “T-shaped” edge-to-face structural motif. On the other hand, one of the most promising materials in the organic field-effect transistor realizing the highest mobility is rubrene, which affords another characteristic packing motif, so-called “pitched π-stack”, where the long-molecular axis are inclined so as to form partial π-stacking with end-to-face intermolecular interaction. Although control and prediction of the solid state structures from the molecular level are regarded as formidable task, we recently found that simple methylthionation on a series of thienoacenes selectively induces the rubrene-like “pitched π-stack” in the solid state. These results suggest that a proper molecular modification can pave the way to “artificial rubrene”, i.e., packing structure control of thienoacenes for high-performance organic semiconductors.

Control of packing structures of thienoacene-based organic semiconductors